Medovate today announced that the SAFIRA® Palm Operator has officially been launched in the US market as part of the FDA cleared SAFIRA® product range.
CAMBRIDGE, UNITED KINGDOM, January 31, 2022 /EINPresswire.com/ -- Medovate – a pioneering medical device development company – today announced that the SAFIRA® (SAFer Injection for Regional Anesthesia) Palm Operator has officially been launched in the US market as part of the FDA cleared SAFIRA® product range.
The company hopes that the addition of a hand operator option to the SAFIRA® product range will offer anesthesiologists who practice regional anesthesia greater choice and versatility when using the device.
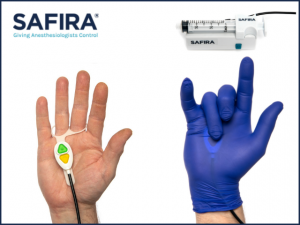
The Palm Operator uses the same colour coding as the original SAFIRA® foot pedal operator, which remains a key part of the product range. Both operator options give the anesthesiologist full control of the injection process during regional anesthesia so they do not require an assistant to operate the syringe. The
Palm Operator is ergonomically designed to comfortably fit under a surgical glove, within the palm of a hand, giving anesthesiologists full control of aspiration and infusion during the injection process.Supporting healthcare professionals with a more complete solution to their regional anesthesia needs, the handheld operator will be made available through Medovate’s US distributors as part of their expanded SAFIRA® product offering.
Alan Finnerty, Technology Director at Medovate said: “We are delighted to be extending our Palm Operator offering to clinicians in the US as part of our roll out of SAFIRA®. The additional option will offer anesthesiologists more versatility and choice when using our system. It means we are now able to support healthcare providers with a complete solution to their regional anesthesia needs with both hand and foot operators. We are thrilled to celebrate this next step in our FDA cleared SAFIRA® offering for the US.”
Developed with clinicians working in the UK’s NHS to promote safer injection during peripheral nerve block procedures, SAFIRA® transforms regional anesthesia into a one-person procedure, giving the anesthesiologist full control of the injection at all times. It also includes a built-in safety mechanism to limit injection pressure helping to reduce the risk of nerve damage and promote patient safety.
The system consists of 3 separate components – a proprietary syringe, a driver and an operator. The SAFIRA® operator is available in two variants: a foot pedal operator that sits on the floor during a procedure, and the SAFIRA® Palm Operator – a hand worn operator that is worn under a surgical glove.
With an estimated 10 million regional anesthesia blocks carried out each year in the US, the addition of a further operator option for SAFIRA® marks a significant milestone for Medovate and means they can expand their product offering to clinicians across the country.
SAFIRA® was launched in the United States in 2020 and Mercury Medical is the primary distribution partner. Medovate have also signed a co-promotional agreement with Konica Minolta Healthcare Americas Inc. to promote best practice in ultrasound guided regional anesthesia across the US.

No comments:
Post a Comment