Independent Study and Bioanalysis Suggests More Effective Nerve Repair and Faster Wound Healing
TORONTO, CANADA AND HAIFA, ISRAEL, August 13, 2025 /EINPresswire.com/ -- NurExone Biologic Inc. (TSXV: NRX) (OTCQB: NRXBF) (FSE: J90) (“NurExone” or the “Company”) today announced that an independent study showed that exosomes produced by NurExone outperformed a recognized commercial industry standard in areas that strongly support key healing tasks including nerve repair, wound repair, calming the immune system and rebuilding tissue. This first independent analysis highlighted the broad potential of NurExone’s exosomes for both therapeutic and aesthetics markets.
“This data confirms that the naïve
“Our exosomes carry complex cargo with diverse therapeutic potential, simultaneously being effective in neuroprotection and reduction in inflammation,” said Dr. Tali Kizhner, Director of R&D of NurExone Biologic. “This combination is especially powerful in the nervous system, where inflammation usually prevents healing. The benchmarking analysis confirms that our exosomes naturally carry a significant amount of the molecular signals needed to create the conditions required for meaningful nerve regeneration.”
TAmiRNA, an ISO 13485‑certified molecular‑diagnostics laboratory, performed a comprehensive analysis of the EV microRNA cargo of NurExone’s exosomes and compared the results with benchmark exosomes from a commercial reference source. Based on TAmiRNA’s data, bioinformatic analyses showed that NurExone’s exosomes are enriched with microRNAs that support key healing tasks.
Importantly, these exosomes were produced from NurExone’s proprietary master cell bank, whose cells are maintained under rigorously controlled environment ensuring that every production batch delivers high-performance exosomes. This reproducibility is essential for clinical translation and future patient use.
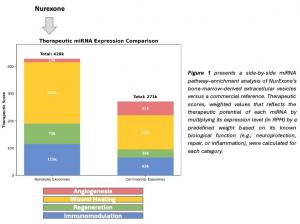
Figure 1 suggests that NurExone’s naïve exosomes outperform the commercial reference across every pathway assessed: they exhibit ≈1.8-fold higher neurological potential, nearly double the anti-inflammatory activity, and a full two-fold increase in both tissue-regeneration and wound-healing signals. This multi-modal, high-potency profile positions the exosomes as a versatile platform for therapeutic nerve repair as well as for aesthetic regenerative and longevity applications.
The results provide strong, third-party validation of NurExone’s core technology and highlight its potential across a broad range of regenerative-medicine applications.
About TAmiRNA GmbH
Vienna‑based TAmiRNA is an ISO 13485‑certified molecular‑diagnostics laboratory whose CE‑marked biomarker kits and miND® small‑RNA sequencing platform provide industry‑leading, ISEV‑compliant exosome analytics. Its extensive extracellular‑vesicle reference database and bench‑to‑algorithm workflow deliver regulatory‑grade, reproducible insights for precision‑medicine programs.
About NurExone
NurExone Biologic Inc. is a TSX Venture Exchange (“TSXV”), OTCQB, and Frankfurt-listed biotech company focused on developing regenerative exosome-based therapies for central nervous system injuries. Its lead product, ExoPTEN, has demonstrated strong preclinical data supporting clinical potential in treating acute spinal cord and optic nerve injury, both multi-billion-dollar marketsi . Regulatory milestones, including obtaining the Orphan Drug Designation, facilitates the roadmap towards clinical trials in the U.S. and Europe. Commercially, the Company is expected to offer solutions to companies interested in quality exosomes and minimally invasive targeted delivery systems for other indications. NurExone has established Exo-Top Inc., a U.S. subsidiary, to anchor its North American activity and growth strategy.
For additional information and a brief interview, please watch Who is NurExone?, visit www.nurexone.com or follow NurExone on LinkedIn, Twitter, Facebook, or YouTube.

No comments:
Post a Comment